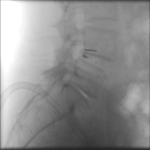
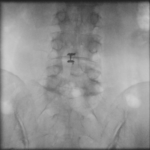
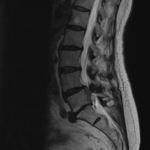
The Barricaid anular closure device is a revolutionary medical device, implanted to block an annular defect. Implanting this device makes it possible to avoid a more aggressive discectomy for patients who at high risk of experiencing recurrent disc herniation. Dr. Jonathan Stieber, a top spine surgeon in NYC, performed the first Barricaid anular closure device surgery in the United States and is uniquely qualified to perform this advanced spinal surgery.
Ruptures or tears in the annulus fibrosus in a disc leads to moderate-to-severe lower back pain. This device was developed ease suffering, improve surgical outcomes, and prevent a recurrence of herniation, and avoid the need for an aggressive removal of the remaining disc nucleus.
How does the Barricaid anular closure device work?
The Barricaid anular closure device is implanted after a microdiscectomy (a procedure to resolve the pain and discomfort associated with a lumbar herniated disc). The device anchored to the adjacent vertebrae, in a minimally-invasive spine surgery, performed on an outpatient basis by Dr. Jonathan Stieber.
What is the recovery time?
Some post-op activity modification will be necessary during your recovery. Most people who have had spinal surgery with this new technique can return to their usual activities within a few weeks.
What does Barricaid do?
Intrinsic Therapeutics developed the Barricaid device to close large defects in the annulus, the outer part of a spinal disc.
- Barricaid is used after discectomy, which is a surgery to remove lumbar herniated disc material that may be pressing on a nerve root or the spinal cord, causing pain.
- The procedure has proven to prevent recurrent disc herniation.
- The Barricaid anular closure device can reduce the need for additional spine surgeries.
The Barricaid anular closure device was clinically studied for over eight years, is FDA-approved, and through clinical trials has been proven to be very effective in reducing re-herniation in high-risk patients.
Am I a good candidate for Barricaid anular closure device surgery?
A disc herniation occurs when the inner walls of a disc in your spine pushes through the outer walls of the disc, causing pain. This leaves a hole that increases the risk of a patient suffering a return of the painful condition. You may be a good candidate for this groundbreaking treatment if:
- You are an adult with a fully formed spine
- You have a history of recurrent herniation
- You are at risk of recurrent disc collapse
- You have “tall” discs in relation to the size of your anular defect
Patients who have small annular defects have a much lower risk of recurrent injury and issues, so they are not appropriate candidates for this procedure.
Why choose Dr. Jonathan Stieber?
In 2015, Dr. Stieber had the opportunity to work with surgeons in Germany, Austria, and Belgium on the use of this revolutionary implant. He was honored to perform the first FDA-approved Barricaid anular closure device surgery in the United States. After the successful and productive collaboration with his European counterparts, he is very pleased to now offer this advanced treatment to his patients.
Given that recurrent disc herniation occurs in ten to fifteen percent of patients who undergo primary lumbar disc surgery, Dr. Stieber may recommend that a Barricaid anular closing device be placed during your microdiscectomy procedure. He has seen firsthand how this groundbreaking surgical technique can help his patients avoid the risk of recurrent back injury and alleviate pain. For more information and to schedule a consultation, contact us.
International Spinal News
“We’re excited to be the first in the USA to perform discectomy with implantation of the Barricaid, addressing what has been a long unmet need in the field of spine surgery,” says Dr. Stieber.
Press Release
Dr. Stieber is mentioned in an NYU Lagone Health press release regarding the newly-approved Barricaid Anular Closure Device that reduces the recurrence of disc herniation. Read this here.